
When we say 'elementary', scientists mean particles that cannot be broken down into even smaller particles. Particle generationsĪccording to the Standard Model, there are three families of elementary particles. Scientists needed a way of organizing and streamlining it all, and their answer to this was to create the Standard Model, which is the crowning glory of the cumulative work of the physics community of that era. By the 1960s, with the advent of fledgling particle accelerators, hundreds of particles were being discovered, and the scientific picture was becoming very complicated indeed. The muon was identified in 1936 by Anderson and Seth Neddermeyer (opens in new tab), and then the pion was discovered in 1947 (opens in new tab) by Cecil Powell. In 1932 the American physicist Carl Anderson discovered the positron (opens in new tab), which is the antimatter equivalent of an electron. The existence of the photon was already known, so technically that was a fourth particle. So, the picture of particle physics in the early 1930s seemed relatively straightforward - atoms were made of two kinds of 'nucleons', in the guise of protons and neutrons, and electrons orbited them.īut things were already quickly starting to become more complicated. The proton's neutrally charged partner, the neutron, was identified in 1932 by James Chadwick (opens in new tab) at Cambridge, who also won the Nobel Prize. That particle was the proton (opens in new tab), which gives the atomic nucleus its positive charge. Further experimentation by Rutherford in 1919–20 found that an alpha particle fired into air could knock a positively charged particle out of a nitrogen atom in the air, turning it into carbon in the process. Rutherford interpreted this as meaning that atoms contained a lot of empty space that the alpha particles were passing through, but that their positive charge was concentrated in a nucleus at their center, and on the occasions, an alpha particle hit this nucleus dead on, it was scattered. (Image credit: Wikimedia Commons/Kurzon) (opens in new tab) Some of the alpha particles passed right through the atoms in the foil, while others were scattered left and right and a small fraction bounced right back.Īn illustration of the gold foil experiment. Then, in 1911, Hans Geiger and Ernest Madsen, under the supervision of the Nobel Laureate Ernest Rutherford (opens in new tab) at the University of Manchester, performed their famous 'gold foil' experiment, in which alpha particles (helium nuclei) were fired at a thin gold foil.
The first subatomic particle to be identified, in cathode rays (opens in new tab), was the negative electron in 1897 by the British physicist and subsequent Nobel Prize winner, J. By the 1880s, it was becoming apparent that there were positively and negatively charged particles produced when gasses are ionized, and that these particles must be smaller than atoms, which were the smallest known structures at the time.
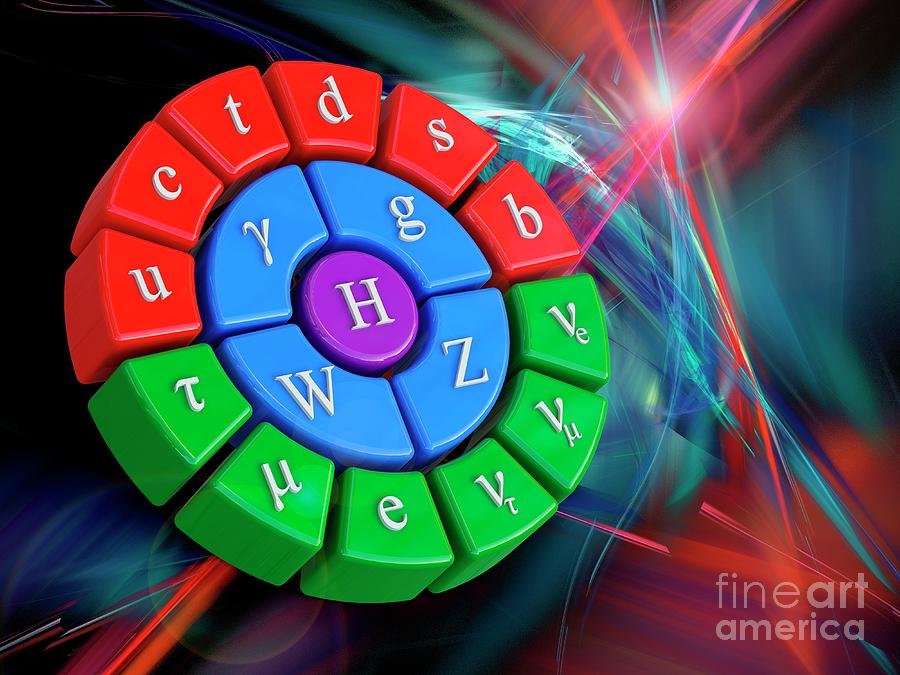
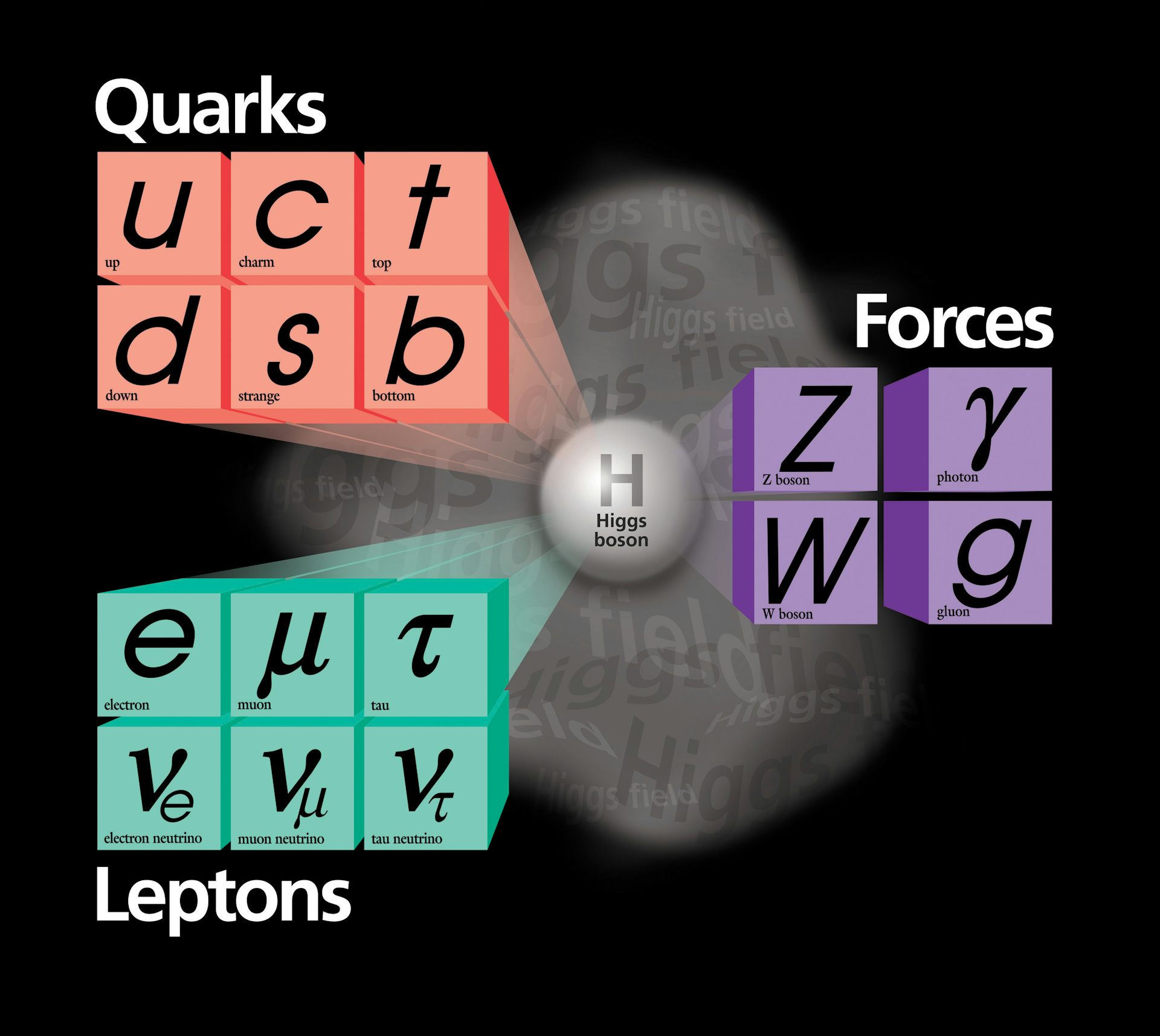
The Standard Model was drawn together in the 1960s and early 1970s from the work of a cadre of pioneering scientists, but in truth its origins extend back almost 100 years earlier.


 0 kommentar(er)
0 kommentar(er)
